Pharmaceutical packaging must be optimally sealed to prevent foreign substances from entering and it must also follow all legal guidelines on counterfeit protection and traceability. When it comes to the production of pharmaceutical packaging, priority is given to the protection of the high-quality ingredients and formulations while maintaining complete functionality. Based in Germany, Uhlmann Pac-Systeme GmbH & Co. KG is one of the world’s leading suppliers of pharmaceutical packaging. Uhlmann’s product portfolio includes machines for all process steps: from blister machines and bottling lines to cartoners and end-of-line packaging machines. This range is complemented by extensive services for the systems’ entire life cycle.
New knowledge on the reproducibility of manufacturing processes
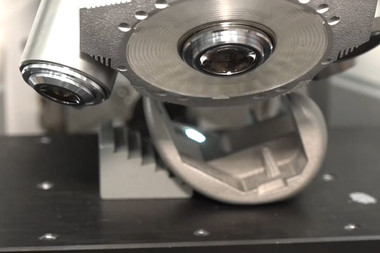
When manufacturing special pharmaceutical machines, the focus is to implement professional quality assurance processes at all stages of production. Uhlmann use the high-resolution measurement system InfiniteFocus to test the machine components and the packaging products they fabricate. "Bruker Alicona allows us to gain new knowledge on the reproducibility of our manufacturing processes. Besides this, the measurements also enable us to verify supplier specifications for purchased parts, predict the functional behaviour of products and carry out cause analyses on the functionality of products”, says Matthias Obert, member of Uhlmann’s Quality Management team.
Roughness measurements to monitor manufacturing of blister machines
Obert explains the process monitoring during serial production, using the manufactured blister machines as an example. "We use Bruker Alicona to carry out monitoring measurements in the manufacturing process and to verify the geometry and surface properties of individual components.” Blister machines are used to produce blister packaging, better known as "blister packs”. These packs contain the pre-sorted medicine and are hygienically sealed using plastic or aluminium composite foils. Each separate drug unit has its own "cup”, from which the tablet or capsule can be popped out with your fingers.
Sa, Sz, Sk roughness parameters verify functionality
The process steps of a blister packaging machine include, first and foremost, the shaping of the cup-shaped depressions into the base, which is made of aluminium foil. The product, tablets or capsules, are then filled into the cups. In a next step, a cover film is fed through the sealing station and placed on the base foil. The cover film is warmed up by means of hot plates to enable it to mould to the base foil, thus enclosing the product in the cup-shaped depression. In order to prevent sticking to the hot plates and to ensure optimum heat distribution on the hot plates, Uhlmann implement the measuring system to determine surface parameters (Sa, Sz) and material parameters (Sk, Spk). Obert:”Surfaces with similar Sa values can have completely different structures. Often, one can only provide a sound statement about the functional behaviour of the surface after evaluating the material ratio parameters.”
"Dimensional measurement gives us essential information”!
Sealing rollers made of tool-grade steel or high-quality aluminium are used for bonding the film to the foil. The contact surfaces of the rollers have inclined surfaces and tips, i.e. pyramid-shaped corrugation. The distribution density of these "pyramids” dictates how deep the tips penetrate in the foil composite and thus provides a contact face adapted to the surface pressing requirements. "Before we switched to the measuring technology of Bruker Alicona, we could not measure these corrugated geometries. The analysis of the pyramid stump dimensions, such as the angle, height and distance to each other, gives us indispensable information.”
Impact of shape, roughness and color information
Once the sheet is sealed air-tight, each tablet is enclosed by two longitudinal sealing gutters and two transverse sealing gutters. The seal shape of the blisters is checked in the course of the packaging process. The shape measurement and color evaluation enable Uhlmann to check the homogeneity of the impression and lacks gutter width and height. After sealing the carrier foil and cover film, it is possible to emboss specific safety features (e.g. batch number) permanently on the blister reel. The serial numbers are punched in using die stamps. Uhlmann use InfiniteFocus to ensure the correct letter height of the die stamp, measure the surface roughness and perform a visual inspection.
Simple handling and extensive applicability
The cut areas of the punched blister packages are then evaluated. "About Bruker Alicona, we particularly appreciate the ease of use of the system and its extensive applicability. The color-coded height representation comes in very helpful to communicate surface properties easily and intelligibly to other interfaces in the company", explains Obert.
Why tactile measurement is no longer suitable
In order to ensure the quality of the production process of the blister machines, Uhlmann also require information on the slip properties of the tablets and capsules. To do this, one needs to know the surface roughness of the medicine. Tactile measuring methods are not suitable for this application because the surface of the measuring object can alter or get damaged in direct contact with the medicine. Bruker Alicona helps determine surface characteristics of the tablets (e.g. Sa and Sz) without touching the surface.